Artificial Blood Cells: Revolutionizing Transfusion Medicine
Key Takeaways
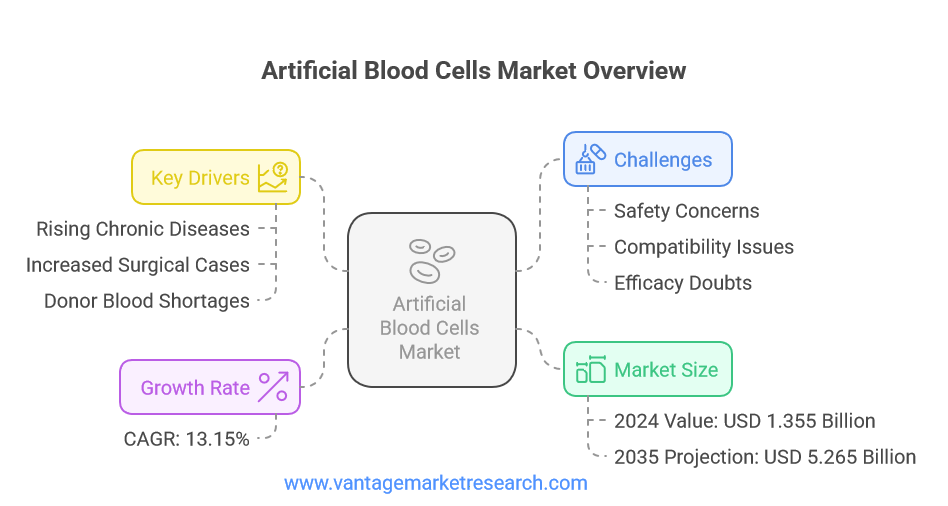
- The global Artificial Blood Cells Market is valued at USD 1.355 Billion in 2024 and is projected to reach USD 5.265 Billion by 2035, growing at a CAGR of 13.15%.
- Artificial blood cells are synthetic substitutes designed to replicate key functions of natural red blood cells, primarily oxygen and carbon dioxide transport.
- Market growth is driven by rising chronic diseases, increased surgical and trauma cases, and limitations in donor blood supply.
- Hemoglobin-based and perfluorocarbon (PFC) products dominate the market, with emerging nanotechnology applications on the horizon.
- North America leads the market, while Asia Pacific shows significant growth potential and Europe benefits from robust healthcare infrastructure.
- Key challenges include safety, efficacy, regulatory hurdles, and competition from traditional blood products.
- Strategic developments focus on innovation, partnerships, and research, with commercialization expected to accelerate in the coming decade.
Overview of the Artificial Blood Cells Market
The Artificial Blood Cells Market is at the forefront of a transformative era in transfusion medicine. As of 2024, the market is valued at USD 1.355 billion, with projections indicating a substantial rise to USD 5.265 billion by 2035. This impressive growth, at a CAGR of 13.15% between 2025 and 2035, underscores the increasing demand for alternatives to traditional blood transfusions. Artificial blood cells, also known as synthetic blood or blood substitutes, are engineered to mimic the essential functions of natural red blood cells, particularly the transport of oxygen and carbon dioxide throughout the body. These products are developed using advanced chemical isolation techniques or recombinant biochemical technologies, offering a promising solution to the perennial challenges of blood shortages and transfusion-related complications.
Despite the market’s potential, commercialization has been slower than anticipated. Several artificial blood cell products are in advanced clinical trial phases, but widespread adoption is hindered by ongoing concerns regarding safety, compatibility, and efficacy. Nevertheless, the market’s projected growth trajectory is fueled by a confluence of factors, including the rising prevalence of chronic diseases, an increase in surgical and trauma cases, and the persistent limitations of donor blood supply. As research and development efforts intensify, and as regulatory pathways become clearer, the artificial blood cells market is poised to play a pivotal role in the future of healthcare, offering new hope for patients and healthcare providers alike.
Key Factors Influencing Market Growth
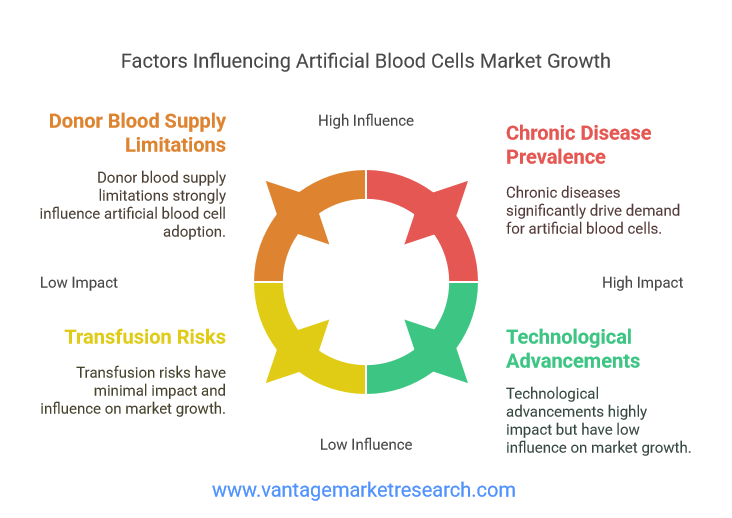
The artificial blood cells market is being shaped by several critical factors that are driving demand and shaping the industry’s future. One of the most significant drivers is the global rise in chronic diseases such as cancer, cardiovascular disorders, and anemia. These conditions often necessitate frequent blood transfusions, placing immense pressure on existing blood supplies. Additionally, the increasing number of surgical procedures and trauma cases worldwide has further exacerbated the demand for safe and reliable blood substitutes. In emergency situations, where immediate access to compatible donor blood is not always possible, artificial blood cells offer a potentially life-saving alternative.
Another key factor influencing market growth is the inherent limitations of donor blood supply. Blood donations are subject to fluctuations due to donor availability, seasonal trends, and unforeseen events such as pandemics or natural disasters. Moreover, the risk of transfusion-transmitted infections, immunological reactions, and blood type incompatibility remains a persistent concern. Artificial blood cells, with their standardized composition and reduced risk of contamination, present a compelling solution to these challenges. Furthermore, advancements in biotechnology and nanotechnology are enabling the development of next-generation artificial blood products with enhanced functionality and safety profiles, further fueling market expansion.
Types of Artificial Blood Products
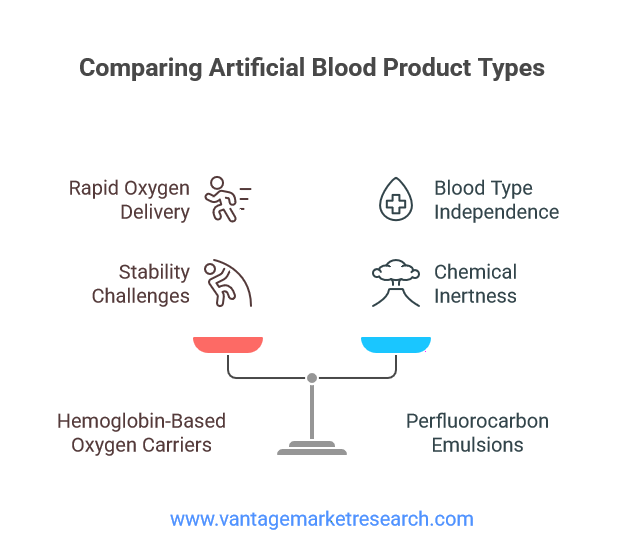
The artificial blood cells market encompasses a diverse range of products, each with unique features and advantages. The two primary categories are hemoglobin-based oxygen carriers (HBOCs) and perfluorocarbon (PFC) emulsions. Hemoglobin-based products are designed to replicate the oxygen-carrying capacity of natural red blood cells by utilizing purified or recombinant hemoglobin molecules. These products offer rapid oxygen delivery and are particularly useful in situations where immediate transfusion is required. However, challenges related to stability, toxicity, and vascular reactivity have limited their widespread adoption.
Perfluorocarbon (PFC) products, on the other hand, are synthetic compounds capable of dissolving and transporting large amounts of oxygen and carbon dioxide. PFCs are chemically inert and can be emulsified for intravenous administration, making them suitable for a variety of clinical applications. Their ability to function independently of blood type compatibility is a significant advantage, especially in emergency and battlefield settings. In recent years, emerging synthetic and nanotechnology-based approaches have shown promise in enhancing the performance and safety of artificial blood products. Nanoparticle-based carriers, for example, can be engineered to mimic the size, shape, and surface properties of natural red blood cells, improving circulation time and reducing immunogenicity. As research in this field progresses, the range and efficacy of artificial blood products are expected to expand significantly.
Regional Market Insights
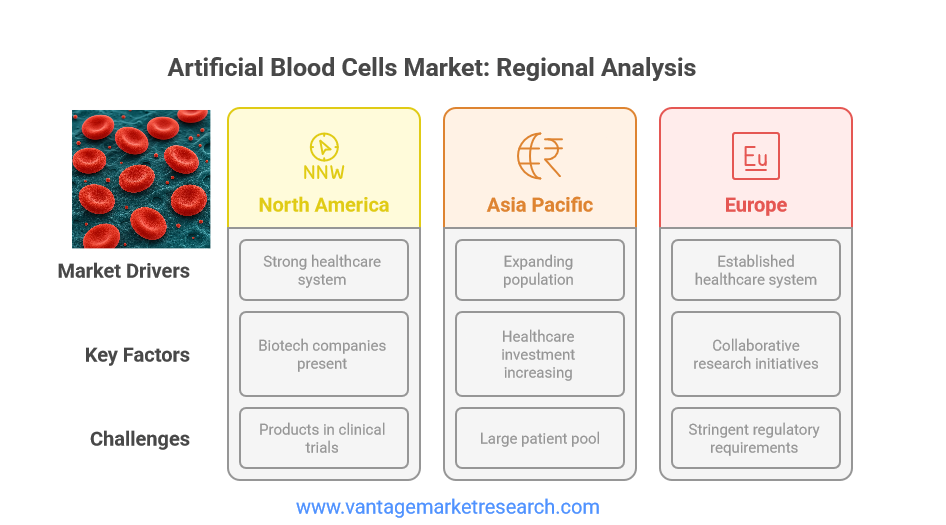
Geographically, the artificial blood cells market exhibits distinct trends and growth patterns across different regions. North America currently dominates the market, driven by a robust healthcare infrastructure, significant investment in research and development, and a high prevalence of chronic diseases. The presence of leading biotechnology companies and favorable regulatory frameworks further contribute to the region’s market leadership. The United States, in particular, has been at the forefront of artificial blood research, with several products advancing through clinical trials and regulatory review.
Asia Pacific represents a region of immense growth potential, fueled by a rapidly expanding population, increasing healthcare expenditure, and rising awareness of advanced medical technologies. Countries such as China, India, and Japan are investing heavily in healthcare innovation, creating opportunities for the adoption of artificial blood products. The region’s large patient pool and unmet medical needs make it an attractive market for both established players and new entrants.
Europe, with its well-established healthcare infrastructure and strong regulatory oversight, also plays a significant role in the artificial blood cells market. The region benefits from collaborative research initiatives, government support, and a high standard of medical care. However, stringent regulatory requirements and the need for comprehensive clinical data can pose challenges to market entry and product approval. Overall, regional dynamics are expected to evolve as technological advancements and regulatory harmonization drive global market integration.
Challenges Facing the Market
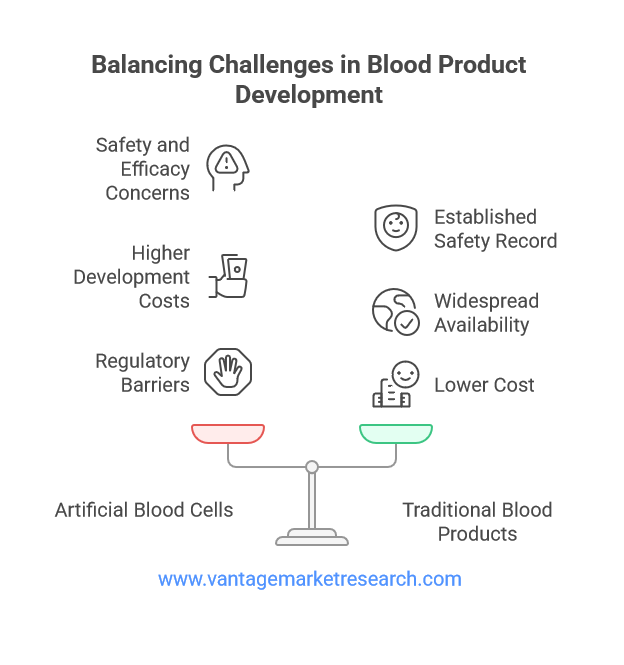
Despite the promising outlook, the artificial blood cells market faces several formidable challenges that must be addressed to ensure long-term success. Safety and efficacy remain paramount concerns, as artificial blood products must demonstrate not only the ability to transport oxygen effectively but also a favorable safety profile with minimal adverse effects. Issues such as hemoglobin toxicity, immune reactions, and vascular complications have been observed in some clinical trials, underscoring the need for rigorous testing and continuous monitoring.
Regulatory barriers and lengthy approval timelines also pose significant obstacles to market growth. The development of artificial blood products involves complex manufacturing processes and stringent quality control measures, necessitating comprehensive preclinical and clinical evaluation. Regulatory agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) require robust evidence of safety, efficacy, and quality before granting approval, which can delay commercialization and increase development costs.
Competition from traditional blood products is another challenge, as donor blood remains the gold standard for transfusion in most clinical settings. The widespread availability, established safety record, and lower cost of donor blood make it difficult for artificial blood products to gain widespread acceptance. Overcoming these challenges will require continued innovation, strategic partnerships, and effective communication of the benefits and safety of artificial blood cells to healthcare providers and patients.
Strategic Developments in the Market
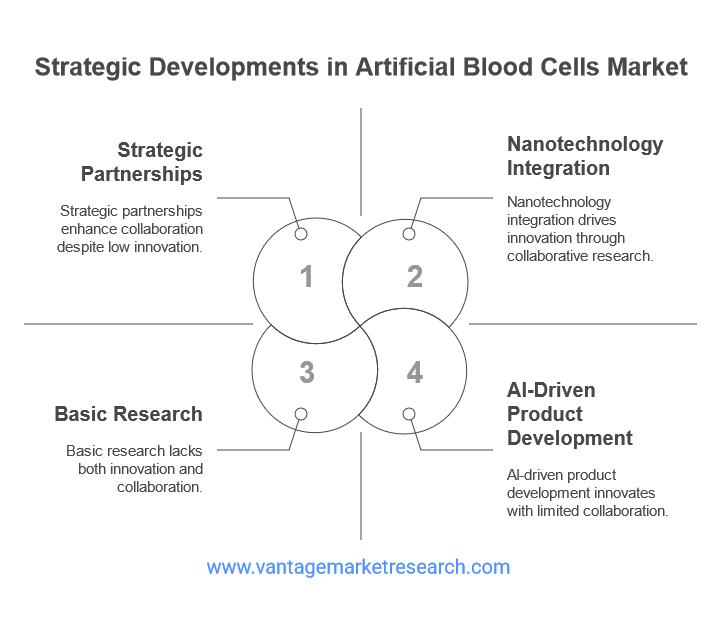
The artificial blood cells market is characterized by a dynamic landscape of innovation, collaboration, and strategic investment. Recent years have witnessed significant breakthroughs in product development, with several companies advancing novel artificial blood products through preclinical and clinical stages. Key players in the market include both established biotechnology firms and emerging startups, each contributing unique expertise and resources to the field.
Strategic partnerships between industry, academia, and government agencies are playing a crucial role in accelerating research and development. Collaborative efforts are focused on optimizing product formulations, improving manufacturing processes, and conducting large-scale clinical trials to generate robust safety and efficacy data. In addition, public and private funding initiatives are supporting the translation of promising research into commercial products.
Innovation remains at the heart of market growth, with ongoing research exploring new materials, delivery systems, and functional enhancements for artificial blood cells. Advances in nanotechnology, for example, are enabling the design of biomimetic carriers with improved circulation time and reduced immunogenicity. The integration of artificial intelligence and machine learning is also facilitating the identification of optimal product candidates and the prediction of clinical outcomes. As the market continues to evolve, strategic developments will be essential in overcoming existing challenges and unlocking the full potential of artificial blood cells.
Future Projections for Artificial Blood Cells
Looking ahead, the future of the artificial blood cells market is marked by optimism and anticipation. Analysts predict that commercialization timelines will accelerate as ongoing clinical trials yield positive results and regulatory pathways become more streamlined. The potential for broader applications in healthcare is significant, with artificial blood cells poised to address critical needs in emergency medicine, surgery, oncology, and chronic disease management.
In the long term, the vision for artificial blood extends beyond simple oxygen transport. Researchers are exploring multifunctional artificial blood cells capable of delivering therapeutic agents, monitoring physiological parameters, and even supporting tissue regeneration. The integration of smart technologies and personalized medicine approaches could further enhance the utility and effectiveness of artificial blood products.
As the market matures, increased investment in research, infrastructure, and education will be essential to ensure the safe and effective adoption of artificial blood cells. Collaboration between stakeholders, including industry, regulators, healthcare providers, and patients, will be key to realizing the full potential of this groundbreaking technology. Ultimately, artificial blood cells have the potential to revolutionize transfusion medicine, improve patient outcomes, and address some of the most pressing challenges in global healthcare.
FAQs
- What factors are driving the growth of the Artificial Blood Cells Market?
- How do Artificial Blood Cells differ from traditional blood products?
- What are the potential applications of Artificial Blood Cells in medical settings?
- What challenges does the Artificial Blood Cells Market face in terms of regulatory approval and public acceptance?
![[Market Research Reports] – Research Google News Blog | VMR.Biz](https://www.vmr.biz/wp-content/uploads/2022/12/logo-removebg-preview.png)