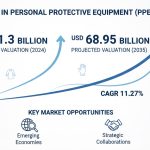
Global Pyrogen Testing Market
As stated in our extensive report, the Global Pyrogen Testing Market accounted for USD 1.2 Billion in 2022 and is projected to reach a value of USD 3.2 Billion by 2030.
The Pyrogen Testing Market pertains to the sector specializing in tests designed to detect and quantify pyrogens in pharmaceutical and biotechnology products. Pyrogens are substances capable of inducing fever when introduced into the body and can be present in various materials used to manufacture drugs, vaccines, and medical devices.
Several crucial variables have propelled constant expansion in the global Pyrogen Testing market in recent years. There is a rising emphasis on guaranteeing the safety and quality of pharmaceutical goods, an expanding incidence of chronic diseases, and a surge in consumer demand for pharmaceutical products. Additionally, stringent government regulations and guidelines about the testing and security of pharmaceutical products have played a significant role in fostering market expansion.
The expansion of the global Pyrogen Testing market is propelled by the growth of the pharmaceutical, biotechnology, and medical devices sectors, augmented by rising investments in research and development and supported by favorable government policies. Nevertheless, stringent regulatory requirements serve as a constraint on market growth. Furthermore, the increasing trend of outsourcing activities within the pharmaceutical industry, particularly in developing nations, presents significant opportunities for market advancement.
Click To Get a Free Sample On the Research Study

Key Factors Influencing Global Pyrogen Testing Market Growth
The growth of the global Pyrogen Testing market can be attributable to the following:
- The production of medical devices and pharmaceuticals is heavily regulated, and severe standards for quality control are enforced. Regulatory authorities like the US FDA and the European Pharmacopoeia have established strict guidelines for {Pyrogen Testing to guarantee the safety and effectiveness. This has increased demand for Pyrogen Testing services and products.
- There is a rising need for secure and effective pharmacological and medical-related goods due to the rise in infectious diseases worldwide. Pyrogen contamination in these products can cause serious health risks, and therefore, the demand for Pyrogen Testing to ensure product safety is increasing.
- Advances in technology have led to the development of more accurate, sensitive, and efficient Pyrogen Testing methods. For example, adopting in vitro Pyrogen Testing techniques, such as the LAL test, has greatly streamlined the procedure and reduced the time it takes to receive findings. These technological advancements have fuelled market growth.
- The pharmaceutical and medical device industries strongly emphasize quality control to meet regulatory standards and ensure patient safety. Pyrogen Testing is essential to quality control processes. The increasing focus on quality control has led to the growth of the Pyrogen Testing market.
- The demand for biologics, such as vaccines, antibodies, and cell therapies, is increasing due to advancements in biotechnology and personalized medicine. These products often require rigorous Pyrogen Testing to ensure safety and efficacy. Additionally, the increasing adoption of medical devices, such as implants and surgical instruments, drives the demand for Pyrogen Testing.
North America Region to Express the Largest Market Growth
The North America has witnessed significant growth in 2022 and is expected to continue its growth trajectory in the coming years. Pyrogen Testing refers to detecting the presence of pyrogens in pharmaceutical products, medical devices, and other biological substances. Pyrogens are compounds that, when ingested, can raise the body’s temperature. In addition, technological advancements have also played a significant role in the growth of the Pyrogen Testing market. Traditional Pyrogen Testing methods, such as the rabbit pyrogen test, have limitations in terms of time, cost, and ethical considerations. As a result, there has been a shift towards alternative methods, such as the Limulus Amoebocyte Lysate (LAL) test, which is based on the clotting reaction of horseshoe crab blood. The LAL test is more sensitive, specific, and quicker than traditional methods, leading to increased industry adoption.
Furthermore, major pharmaceutical and biotechnology companies in North America have contributed to the growth of the Pyrogen Testing market. These companies have stringent quality control measures and regularly test their products for pyrogen contamination. The creation of novel medicines and medical equipment due to the pharmaceutical industry’s increased emphasis on research and development operations has also increased demand for Pyrogen Testing.
Conclusion
In conclusion, the expansion of the Pyrogen Testing market can be ascribed to several critical factors, including the rising need for healthcare products of uncompromised safety, stringent regulatory mandates, ongoing technological innovations, and the Market’s diversification into multiple industries. With a continued emphasis on quality control and safety assurance, the demand for Pyrogen Testing is anticipated to rise in the forthcoming years.
Some of the key players in the Global Pyrogen Testing Market include- BioMerieux SA (France), Charles River Laboratories Inc. (U.S.), Ellab AS (Denmark), Genscript Biotech Corp. (U.S.), Lonza Group AG (Switzerland), Merck KGAA (Germany), Thermo Fisher Scientific Inc. (U.S.), Fujifilm Holdings Corporation (Japan) and others.
![[Market Research Reports] – Research Google News Blog | VMR.Biz](https://www.vmr.biz/wp-content/uploads/2022/12/logo-removebg-preview.png)