Global Vaccine Contract Manufacturing Market
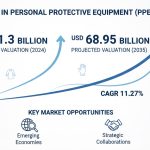
As stated in our extensive report, the Global Vaccine Contract Manufacturing Market accounted USD 3.1 Billion in 2022 and is projected to reach a value of USD 7.4 Billion by 2030.
The Vaccine Contract Manufacturing market refers to the outsourcing of vaccine manufacturing processes by pharmaceutical companies to contract manufacturing organizations (CMOs). These CMOs are specialized companies with the resources, expertise, and capacity to produce vaccines for pharmaceutical companies. The global Vaccine Contract Manufacturing market is proliferating due to various factors, such as the increasing demand for vaccines to combat infectious diseases, the need for large-scale vaccine production for immunization programs, and the complexity of vaccine manufacturing processes. The market is driven by the cost-effectiveness and flexibility offered by contract manufacturing. Outsourcing vaccine production allows pharmaceutical companies to save on capital investments in manufacturing facilities, equipment, and expertise.
One of the key drivers of the market is the growing burden of vaccine-preventable diseases. With the global population and increased travel, the risk of infectious diseases spreading across regions has increased. This has led to a higher demand for vaccines, which has created the need for contract manufacturing of vaccines to meet the growing demand. Additionally, the complexity of vaccine manufacturing processes is another factor driving the contract manufacturing market. Vaccine production involves:
- Specialized technologies and infrastructure
- Stringent quality control measures
- Compliance with regulatory guidelines
Click To Get a Free Sample On the Research Study

These are the primary factors that must be considered to drive the growth of the global market. However, there are challenges in the Vaccine Contract Manufacturing market. Regulatory compliance and quality control are crucial in vaccine manufacturing, and any lapses can severely affect public health. Pharmaceutical companies must carefully select CMOs that meet regulatory standards and have a track record of producing safe and effective vaccines.
Key factors influencing Vaccine Contract Manufacturing Market Growth
The growth of the global Vaccine Contract Manufacturing market can be attributable to the following:
- The rising cases of viral diseases and the need for immunization programs drive the vaccine demand. This is a crucial factor boosting the growth of the Vaccine Contract Manufacturing market.
- Various governments and international organizations promote vaccination programs, especially in developing countries. This has increased vaccine research and development funding, driving the demand for contract manufacturing services.
- Outsourcing vaccine production to contract manufacturers leads to cost and time efficiencies, enabling companies to concentrate on core strengths and cut production expenses. Such a vital element drives the remarkable growth of the market.
- Vaccine production involves various risks, including regulatory compliance, quality assurance, and supply chain management. Contract manufacturers specialize in managing these risks, offering expertise and capabilities to ensure timely and compliant production. This factor is driving the adoption of contract manufacturing services.
- The global vaccine industry is expanding rapidly, driven by increasing demand and the need for vaccines against emerging diseases. Contract manufacturing allows companies to scale up production capacity without significant investments. This has fueled the growth of the Vaccine Contract Manufacturing market.
- Pharmaceutical companies are increasingly partnering with contract manufacturers to leverage their expertise and capabilities. These strategic collaborations enable companies to access specialized manufacturing facilities and resources, accelerating vaccine development and production. Such partnerships are driving market growth.
- Globally, regulatory authorities actively endorse vaccine manufacturing outsourcing to contract manufacturers. They provide comprehensive guidelines and regulations to guarantee the excellence, safety, and efficacy of vaccines produced through this channel. Such robust support significantly fuels the market’s growth.
- Advances in biotechnology and vaccine manufacturing processes have enabled the production of more complex vaccines. Contract manufacturers with expertise in these advanced technologies are in high demand, driving the market’s growth.
North America Region to Lead the Market
North America Vaccine Contract Manufacturing market is getting more significant with maximum market share during the forecast period. This can be attributed to several factors, such as the high prevalence of infectious diseases, increasing vaccination rates, and robust healthcare infrastructure. Furthermore, the presence of major pharmaceutical companies and contract manufacturing organizations (CMOs) in the region has also contributed to its dominance in the market. The rising need for vaccine production and distribution, especially during the ongoing COVID-19 pandemic, has further fueled North America’s demand for contract manufacturing services. Additionally, the region’s favorable regulatory environment and robust intellectual property protection have attracted several global vaccine manufacturers to collaborate with CMOs in North America. With the increasing focus on vaccine development and manufacturing, driven by emerging infectious diseases and the need for immunization campaigns, the North American Vaccine Contract Manufacturing industry is anticipated to grow significantly in the coming years.
Conclusion
The increasing demand for vaccines due to the rising prevalence of infectious diseases and the need for cost-effective manufacturing processes is positively influencing the overall Vaccine Contract Manufacturing market.
Some of the key players in the Global Vaccine Contract Manufacturing Market include- Ajinomoto Co. Inc. (Japan), Albany Molecular Research Inc. (U.S.), Catalent Inc. (U.S.), Cobra Biologics Limited (UK), Cytovance Biologics Inc. (U.S.), Fujifilm Holdings Corporation (Japan), ICON PLC (Ireland), IDT Biologika GmbH (Germany) and others.
![[Market Research Reports] – Research Google News Blog | VMR.Biz](https://www.vmr.biz/wp-content/uploads/2022/12/logo-removebg-preview.png)