Global Medical Device Outsourced Manufacturing Market
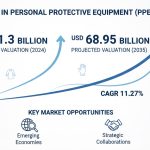
As stated in our extensive report, the Global Medical Device Outsourced Manufacturing Market accounted for USD 29.8 Billion in 2022 and is projected to reach a value of USD 76.8 Billion by 2030.
Outsourcing the manufacturing of medical devices allows companies to focus on their core competencies while benefiting from the expertise and capabilities of specialized contract manufacturers. This helps reduce operational costs, accelerate time-to-market, and improve product quality. The market is witnessing significant growth due to the increasing demand for complex and sophisticated medical devices, such as implantable devices, surgical instruments, and diagnostic equipment. Outsourcing manufacturing processes for such devices helps companies access specialized expertise, advanced technologies, and regulatory compliance, which are essential for developing and producing high-quality devices.
The rising healthcare expenditure and increasing geriatric population also drive the demand for medical devices, thus creating opportunities for contract manufacturers. Moreover, the cost advantages offered by outsourcing manufacturing processes to low-cost regions, such as Asia-Pacific, is further boosting market growth. Another factor contributing to the growth of the Medical Device Outsourced Manufacturing market is the rise of personalized medicine and customized medical devices. Customization and personalization require flexible manufacturing processes, and contract manufacturers are well-suited to provide this level of flexibility.
Click To Get a Free Sample On the Research Study

However, stringent regulatory requirements, intellectual property concerns, and the complexity of supply chain management pose challenges for the market players. Additionally, the COVID-19 pandemic has disrupted the global supply chain, impacting the production and distribution of medical devices, which has further affected the market growth.
Key factors influencing Medical Device Outsourced Manufacturing Market Growth
The growth of the global Medical Device Outsourced Manufacturing market can be attributable to the following:
- The growing need for medical devices, driven by factors such as the rising prevalence of chronic diseases and the aging population, is one of the key factors driving the growth of the Medical Device Outsourced Manufacturing market.
- Outsourcing manufacturing to countries with lower labor and production costs saves medical device companies significantly. This is a major factor driving the outsourcing of medical device manufacturing.
- Outsourcing manufacturing allows medical device companies to access the latest technologies and expertise without investing in expensive equipment and infrastructure.
- Medical devices are subject to strict regulatory requirements for safety and efficacy. Outsourcing manufacturing to specialized contract manufacturing organizations (CMOs) with expertise in regulatory compliance helps medical device companies ensure compliance and navigate complex regulatory processes.
- Outsourcing manufacturing allows medical device companies to focus on their core competencies, such as research and development, marketing, and sales, while leaving the manufacturing processes to specialized CMOs. This helps improve efficiency and speed to market.
- The global nature of the medical device industry, with companies operating in multiple markets, has led to increased manufacturing outsourcing to ensure local market compliance and reduce logistical complexities.
- Outsourcing manufacturing can provide access to specialized expertise and quality control systems, ensuring the production of high-quality medical devices. This is critical in maintaining patient safety and regulatory compliance.
North America Region to Lead the Market
North America Medical Device Outsourced Manufacturing market is getting more significant with maximum market share during the forecast period. North America is the largest region in the Medical Device Outsourced Manufacturing market due to its advanced healthcare infrastructure, technologically advanced manufacturing capabilities, and strong regulatory framework. The region has a highly skilled workforce and many contract manufacturing organizations (CMOs) that provide outsourcing services to medical device companies. Additionally, North America has a high demand for medical devices and many key market players, including established companies and emerging startups. This, combined with the region’s focus on innovation and technological advancements, contributes to North America’s dominance in the Medical Device Outsourced Manufacturing market.
Conclusion
Increasing demand for complex and sophisticated medical devices, such as implantable devices, surgical instruments, and diagnostic equipment, positively influences the overall Medical Device Outsourced Manufacturing market.
Some of the key players in the Global Medical Device Outsourced Manufacturing Market include- SGS SA (Switzerland), Laboratory Corporation of America Holdings (U.S.), Euro fins Scientific (Luxembourg), Pace Analytical Services Inc. (U.S.), Intertek Group PLC (UK), WuXiAppTec (China), North American Science Associates LLC (U.S.), TUV SUD (Germany), Sterigenics U.S. LLC (GTCR LLC) (U.S.), Charles River Laboratories (U.S.) and others.
![[Market Research Reports] – Research Google News Blog | VMR.Biz](https://www.vmr.biz/wp-content/uploads/2022/12/logo-removebg-preview.png)