Global Pharmaceutical Sterility Testing Market
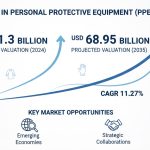
As stated in our extensive report, the Global Pharmaceutical Sterility Testing Market accounted for USD 1.3 Billion in 2022 and is projected to reach a value of USD 3.15 Billion by 2030.
Sterility testing is a crucial step in the pharmaceutical manufacturing process to ensure the safety and efficacy of drugs. It involves testing the absence of viable microorganisms in pharmaceutical products and manufacturing environments. The increasing demand for pharmaceutical products, stringent regulatory requirements, and growing emphasis on quality control are the critical drivers for the growth of the Pharmaceutical Sterility Testing market. Additionally, increasing drug approvals and new product launches further contribute to market growth. With the rising demand for pharmaceutical products, the need for sterility testing has also grown significantly. This is driving the growth of the Pharmaceutical Sterility Testing market.
Moreover, the growing awareness about the significance of sterility testing in drug manufacturing and the increasing focus on patient safety is also responsible for market growth. Governments and regulatory authorities worldwide have implemented strict guidelines and regulatory laws to ensure the safety and efficacy of pharmaceutical products, further fueling the demand for sterility testing. Technological advancements in sterility testing methods additionally support the market. The introduction of automated sterility testing systems and rapid microbiology methods has improved the efficiency and accuracy of the testing process.
Click To Get a Free Sample On the Research Study

These are the primary factors that must be considered to propel the growth of the global Pharmaceutical Sterility Testing market. However, the high cost of sterility testing systems and the stringent regulatory requirements for their validation are expected to hinder market growth somewhat. The COVID-19 pandemic has also disrupted the pharmaceutical product supply chain, affecting the overall market growth.
Key factors influencing Pharmaceutical Sterility Testing Market Growth
The growth of the global Pharmaceutical Sterility Testing market can be attributable to the following:
- With the rise in chronic diseases and infectious diseases, there is a growing demand for pharmaceutical products. Sterility testing is essential to ensure these products are free from microbial contamination, thus driving the market growth.
- Regulatory bodies worldwide, such as the FDA and European Pharmacopoeia, have established strict guidelines and regulations for the testing and approval of pharmaceutical products. Sterility testing is crucial in the approval process, increasing demand for sterility testing services.
- The biopharmaceutical market is experiencing significant growth, driven by biotechnology and genetic engineering advancements. Biopharmaceutical products, such as vaccines, recombinant proteins, and monoclonal antibodies, require sterility testing to ensure their safety and efficacy.
- Pharmaceutical manufacturers are increasingly focusing on quality management procedures to ensure the safety and efficacy of their products. Sterility testing helps identify potential microbial contamination in manufacturing, helping companies maintain quality standards.
- The introduction of advanced technologies, such as rapid sterility testing methods, automated systems, and molecular-based techniques, has improved the efficiency and accuracy of sterility testing. These advancements drive market growth by reducing testing time and improving overall productivity.
- The importance of sterility testing in pharmaceutical manufacturing is being recognized globally. The demand for sterility testing services is increasing with growing awareness among pharmaceutical companies, healthcare professionals, and consumers.
- Many pharmaceutical companies outsource their testing needs to specialized laboratories to reduce costs and improve efficiency. This trend is driving the growth of the sterility testing market, as these outsourcing laboratories specialize in providing high-quality testing services.
North America Region to Lead the Market
North America Pharmaceutical Sterility Testing market is getting more significant with maximum market share during the forecast period. This can be attributed to various factors such as a well-developed healthcare infrastructure, increased research and development activities, and the high demand for quality pharmaceutical products. Additionally, stringent regulatory requirements and the need for sterility assurance in drug manufacturing processes are driving the market in this region. The North American market is expected to continue its dominance in the coming years, supported by technological advancements and increasing investments by pharmaceutical companies in sterility testing. Furthermore, the region’s strong focus on patient safety and quality control practices is further fueling the growth of the Pharmaceutical Sterility Testing market in North America.
Conclusion
Rising demand for pharmaceutical products and the need for sterility testing are positively influencing the overall Pharmaceutical Sterility Testing market.
Some of the key players in the Global Pharmaceutical Sterility Testing Market include- Pacific Biolabs (U.S.), STERIS (U.S.), Boston Analytical (U.S.), Nelson Laboratories LLC (U.S.), Sartorius AG (Germany), SOLVIAS AG (Switzerland), SGS SA (Switzerland), Laboratory Corporation of America Holding (U.S.), Pace Analytical (U.S.) and others.
![[Market Research Reports] – Research Google News Blog | VMR.Biz](https://www.vmr.biz/wp-content/uploads/2022/12/logo-removebg-preview.png)